Thirteen among India’s top-20 selling antibiotic cocktails are classified by the World Health Organisation as neither evidence-based nor recommended, according to a new study that has found dozens of unapproved or banned formulations on sale.
The study found that 229 (58 per cent) of 395 fixed-dose combinations (FDCs) of antibiotics sold across the country were on the WHO’s “not recommended” list.
India’s drug regulatory authority has banned 37 of the 229 formulations, but approved 63.
The findings raise questions about how the Central Drugs Standard Control Organisation (CDSCO) — the regulatory authority — approved formulations considered by the WHO as lacking evidence and why it has not enforced its own bans, researchers said.
“Unapproved and banned (antibiotic) combinations must be removed from the market,” the health researchers said in their study published in the Bulletin of the World Health Organisation, while also flagging the need for “greater transparency” in the CDSCO’s approval process.
An FDC contains two or more medicines packaged into a single pill or tablet. The WHO in 2019 had introduced a list of “not recommended” antibiotic FDCs noting that their use was “not evidence-based, nor recommended in high-quality international guidelines”.
The 13 “not recommended” FDCs accounted for nearly 38 per cent of the country’s total antibiotic combination sales during 2020, according to the study by a six-member team that analysed drug sales data from 10,000 stockists from 30 regions across the country.
The findings suggest that patients across India continue to be exposed to questionable FDCs despite the CDSCO’s efforts to ban unapproved FDCs since 2016.
“What these findings appear to reflect is impunity, a disregard of patients’ health interests,” said Abir Saraswat, a senior dermatologist in Lucknow who is among physicians spearheading campaigns against what they view as irrational FDCs, but was not associated with the new study.
The team that conducted the study said Indian regulatory authorities should review the evidence for marketed formulations not recommended by the WHO but which have been locally approved, the researchers said.
A query sent by this newspaper to the CDSCO seeking responses to the study’s findings has not evoked a reply.
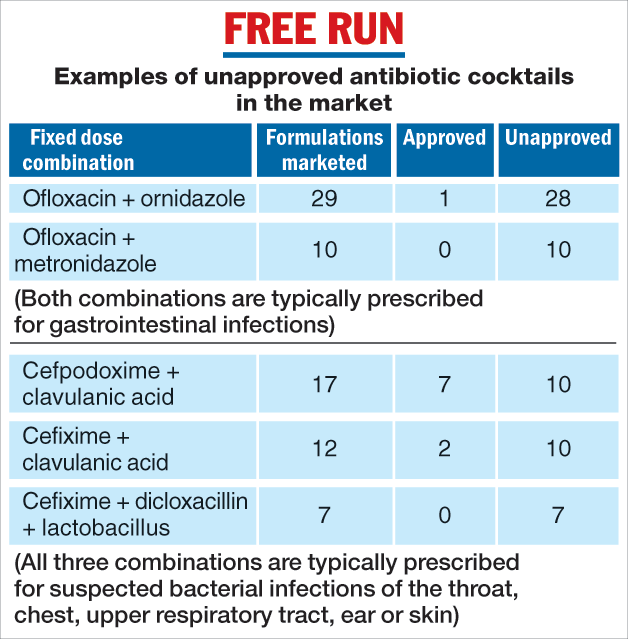
Some examples of antibiotic FDCs not recommended by the WHO but approved in India include ofloxacin with ornidazole and ofloxacin with metronidazole, both typically prescribed for stomach infections, and cefixime with clavulanic acid, cefixime with dicloxacillin, and cefpodoxime with clavulanic acid, all three typically prescribed for lower and upper respiratory tract, skin, or ear infections.
Thirty-seven formulations, accounting for 16 per cent of antibiotic FDC sales, were in the market despite bans by the CDSCO. “There is scope for strengthening enforcement,” said Aashna Mehta, a health economist at the Public Health Foundation of India, New Delhi, a study team member.
The CDSCO approves only specific formulations of FDCs with well-defined dosages of medicines. The study, however, has revealed dozens of unapproved formulations in the market. These include at least 28 formulations of ofloxacin and ornidazole, 10 formulations of cefpodoxime with clavulanic acid, and 10 formulations of cefixime and clavulanic acid.
“Companies create new formulations by simply tweaking dosages — 600mg instead of 500mg — and market them as their exclusive brands,” said a physician who has been tracking FDCs in the country. “Technically, these are unapproved and should not be sold in the market.”
The Centre had in March 2016 notified a ban on over 300 FDCs, including some antibiotic combinations, after an expert panel set up by the CDSCO determined that the FDCs were not supported by evidence and could pose a risk to patients.
However, sections of the pharmaceutical industry challenged the bans in Delhi High Court. A fresh reassessment followed and the Centre issued a fresh ban in January 2019.
But doctors campaigning against what they view as irrational FDCs say little has changed on the ground. “We continue to see completely irrational FDCs, most of them a combination of anti-microbials and steroids, in the market,” said Saraswat.











