Mumbai: Drug firm Lupin has received a warning letter from the US health regulator for its manufacturing facilities in Goa and Indore (Pithampur Unit II) .
"The company has received a warning letter issued by the United States Food and Drug Administration (USFDA) on November 6, 2017 for our formulation manufacturing facilities at Goa and Indore (Pithampur Unit II)," Lupin said in a filing to the Bombay Stock Exchange (BSE) on Tuesday.
The company is deeply disappointed to have received this outcome, it said, adding that while there will be no disruption of product supplies from either of these locations, there is likely be a delay of new product approvals.
Analysts estimate that the two units contribute around 30 per cent to its US sales.
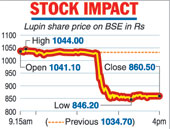
Following the disclosure, Lupin shares plunged nearly 17 per cent, wiping out Rs 7,866 crore from its market valuation. The stock tumbled 16.84 per cent to end at Rs 860.50 on the BSE. During the day, it tanked 18.21 per cent to Rs 846.20, its 52-week low.
On the National Stock Exchange, shares of the company plummeted 16.88 per cent to close at Rs 859.90. The stock was the biggest loser among the bluechips on both the key indices.
The company had earlier received three Form 483 observations for the Goa facility on April 7, 2017 and six Form 483 observations for Pithampur (Unit II) on May 19, 2017, Lupin said and added that it had responded to all the observations.
A Form 483 is issued at the end of an inspection if investigators observe any condition that may violate the Food Drug and Cosmetics Act and related acts.
Lupin, however, did not share the concerns raised by the US health regulator in its warning letter.
"We plan to address the concerns raised by the USFDA expeditiously and will work with the USFDA to resolve these issues at the earliest," Lupin said.
Lupin upholds quality issues with seriousness and remains fully committed to be compliant with cGMP quality standards across all the facilities, it added.